InterAx Biotech supporting the COVID-19 initiative
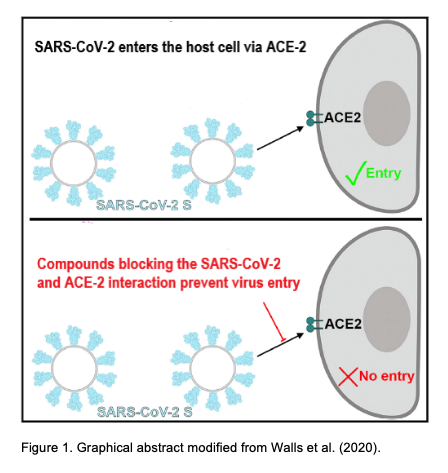
SARS-CoV-2/ACE-2 pseudovirus entry blocking assay
The spike glypcoprotein S of SARS-CoV-2 uses the human Angiotensin Converting Enzyme 2 (ACE2) as an entry receptor and recognizes it with a similar affinity to the 2002–2003 SARS-CoV isolates, which suggests it can spread efficiently in humans, in agreement with the numerous SARS-CoV-2 human-to-human transmission events reported to date (Walls et al., 2020).
InterAx
References
Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell. 2020 Mar 6. pii: S0092-8674(20)30262-2.
Millet JK, Tang T, Nathan L, Jaimes JA, Hsu HL, Daniel S, Whittaker GR. Production of Pseudotyped Particles to Study Highly Pathogenic Coronaviruses in a Biosafety Level 2 Setting. J Vis Exp., 2019 Mar 1;(145).
Millet JK, Whittaker GR. Murine Leukemia Virus (MLV)-based Coronavirus Spike-pseudotyped Particle Production and Infection. Bio Protoc. 2016 Dec 5;6(23). pii: e2035