A Spanish-Swiss Liaison to fight COVID-19
Barcelona, Spain and Villigen Switzerland – 14th April, 2020
Today, Prof. Jana Selent from the Hospital del Mar Medical Research Institute (IMIM) in Barcelona, Spain, and InterAx Biotech in Villigen, Switzerland announce a COVID-19 collaboration, funded by the catalán life sciences and healthcare innovation institution Biocat. The goal of this collaboration is to identify antiviral drugs for the treatment of COVID-19 by combining virtual screening of a 3D-structural database and assessing the effectiveness of these compounds to block viral entry.
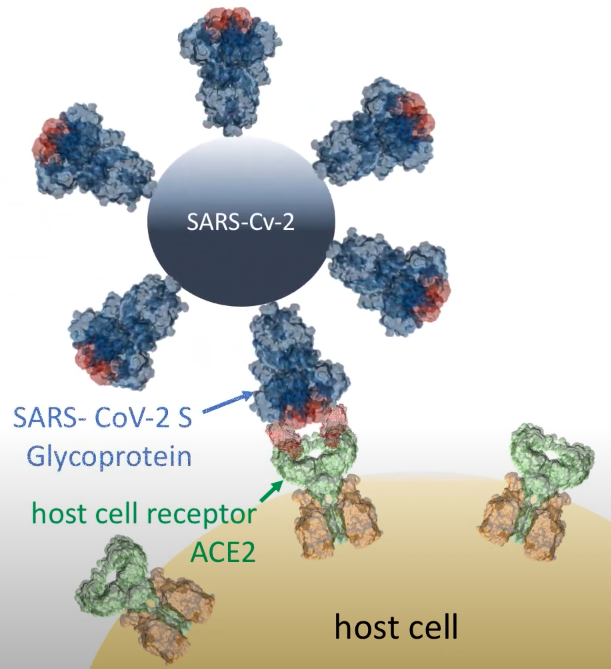
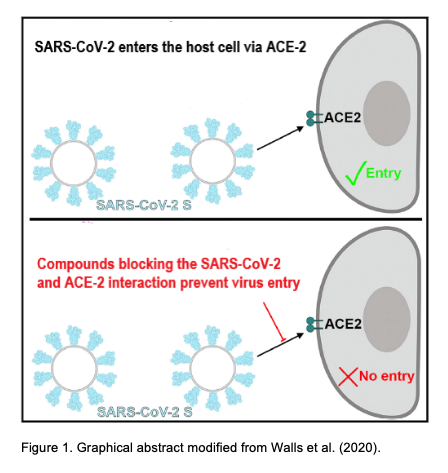
In December 2019, the highly contagious and pathogenic human CoVs (the SARS-CoV-2) appeared in Wuhan (China), which has led to a pandemic with high morbidity and mortality. Prof. Selent and her team have now joined forces with InterAx Biotech to screen for potential antiviral compounds in a virtual 3D-structrual database by using specific computational algorithms and to subsequently test their effectiveness in a SARS-CoV-2 pseudovirus entry blocking assay. The main goal of this project is to identify antiviral drugs for the treatment of COVID-19 by reprofiling existing drugs. Prof. Selent says: ”For this project, we focus on two strategies that interfere with the cell entry of SARS-CoV-2. One is to target the spike glycoprotein S of SARS-CoV-2 and the other one is to tackle the serine protease TMPRSS2. We are excited to team up with InterAx Biotech on testing these compounds in the wet-lab and anticipate that such a dual strategy increases the success rate of discovering effective therapeutic agents against COVID-19.”
The team of InterAx Biotech and Professor Jana Selent’s team hope to test the first antiviral compounds by May 2020.
For further information, please contact:
Dr. Jana Selent, Head of GPCR Drug Discovery Lab | IMIM, 08003, Barcelona, Spain. | Tel. 0034/933160648
Dr. Maria Waldhoer, Chief Science Officer | InterAx Biotech, 5234 Villigen, Switzerland | contact@interaxbiotech.com
Links:
Webpage IMIM: www.imim.es
Webpage Funding agency: www.biocat.cat
Webpage Selent Lab: COVID-19 project progress
Movie: Target modelling